BioCer Entwicklungs-GmbH
Booth number: 4.1H23
www.biocer-gmbh.de
About us
BioCer Entwicklungs-GmbH is a fast-growing medical technology company based in Bayreuth that stands for the highest quality ‘Made in Germany’. Founded in 1998 as a spin-off of the University of Bayreuth and successfully operating in the market since 2010, we pursue the goal of improving the long-term quality of life of patients worldwide.
Our company’s core competencies lie in the development, production and distribution of high-quality medical products. We focus on three central topics: haemostasis, surgical meshes as soft tissue replacement, and the functionalisation of implant surfaces using modern coating technologies.
BioCer products contain neither animal nor human components and meet strict international quality and safety standards.
Our interdisciplinary team combines experts from the fields of process engineering, medicine, natural sciences, materials science and economics. This wealth of expertise and experience enables us to develop pioneering technologies that meet the high demands of medical professionals and the needs of patients alike.
With a worldwide network of distributors, we export our advanced solutions to around 80 countries. As an established Bavarian company, we combine innovative strength with maximum reliability and precision to make a global contribution to improved medical care. Sustainability is a central component of our work: resource-saving procedures and continuous process optimisation contribute to environmentally conscious and responsible medical technology.
Address
Ludwig-Thoma-Str. 36c
95447 Bayreuth
Germany
E-mail: info@biocer-gmbh.de
Phone: +49 921 787770-0
Internet: www.biocer-gmbh.de
Products & Services
BioCer developed the innovative haemostatic products HaemoCer™ and HaemoCer™ PLUS for use in surgical procedures. HaemoCer™ is a 100% plantbased material without any human or animal derived components. HaemoCer™ accelerates the physiological clotting cascade by dehydrating the blood. A robust gel matrix is formed, which is providing a mechanical barrier to further bleeding. HaemoCer™ is absorbed completely within 48 hours. In collaboration with experts, we developed TiO2Mesh™, a surgical mesh implant with a titanium oxide surface coating. TiO2Mesh™ meets the requirements of modern hernia surgery. Due to the biocompatible coating, foreign body reactions are reduced widely, and tissue incorporation and cell ingrowth are improved. TiO2Mesh™ BRA is an innovative surgical mesh implant for breast reconstruction after radical mastectomy. The fabric can be stretched in both directions and is perfectly adapted to the dynamics of human body tissue in terms of elasticity.
HaemoCer™ PLUS
HaemoCer™ PLUS – Natural haemostasis and adhesion prevention
HaemoCer PLUS is a herbal powdered hemostatic agent for supportive haemostasis (bleeding control) and adhesion prevention during surgical procedures. It consists of 100% purified, plant-based starch with no added animal or human components.
HaemoCer PLUS is 100% degraded by the body through amylase within a few days, depending on the amount of material used and the site of application.
TiO2Mesh™
TiO₂Mesh™ und TiO₂Mesh™ light – the titanised mesh implants
The large-pored TiO₂Mesh and TiO₂Mesh light mesh implants are used to reinforce the tissue during hernia surgery. In addition to the optimised surface, they meet all the requirements of modern hernia mesh. TiO₂Mesh meshes offer not only the typical material properties, such as lightweight character and large pores, but also a biocompatible titanium dioxide coating. This titanium oxide layer allows cells to attach to the mesh implant much more easily. In concrete terms, this means that TiO₂Mesh and TiO₂Mesh light heal better in the patient and foreign body reactions are largely avoided.
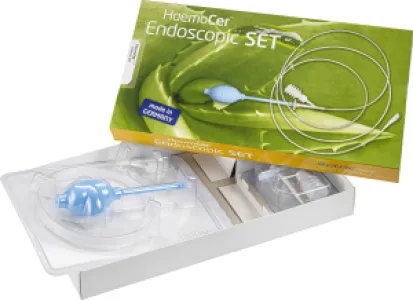
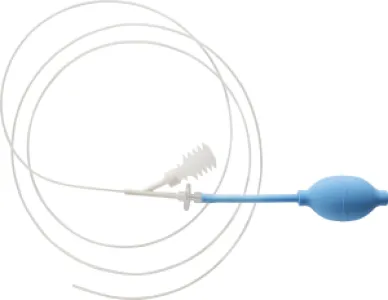
HaemoCer™ Endoscopic Applicator
HaemoCer™ Endoscopic Applicator – Haemostasis during endoscopies
The HaemoCer Endoscopic Applicator is used for haemostasis during endoscopies in the upper and lower gastrointestinal tract. The HaemoCer PLUS haemostatic powder can be applied precisely with the applicator. The applicator has a tube length of 2400 mm, an outer tube diameter of 2.5 mm and a pump ball as a source of compressed air. In addition, the HaemoCer Endoscopic Applicator has a built-in HEPA filter (High Efficiency Particulate Air) for filtering the ambient air.
The HaemoCer Endoscopic Applicator does not require any aids (compressor or similar), making it easy and uncomplicated to use for endoscopies in both the bowel and stomach.
TiO₂Mesh™ BRA
TiO₂Mesh™ BRA + TiO₂Mesh™ BRA light – Soft tissue reinforcement in breast surgery
TiO2Mesh BRA is a surgical mesh implant for the support, reinforcement and bridging of the body’s own soft tissue in reconstructive and medically indicated plastic-aesthetic breast surgery. TiO2Mesh BRA can be stretched in both directions and is perfectly aligned with the dynamics of human body tissue in terms of elasticity. The allogenic mesh implants TiO2Mesh BRA and TiO2Mesh BRA light are available in different sizes.